Our Science
Chronic Pain Treatment Innovation
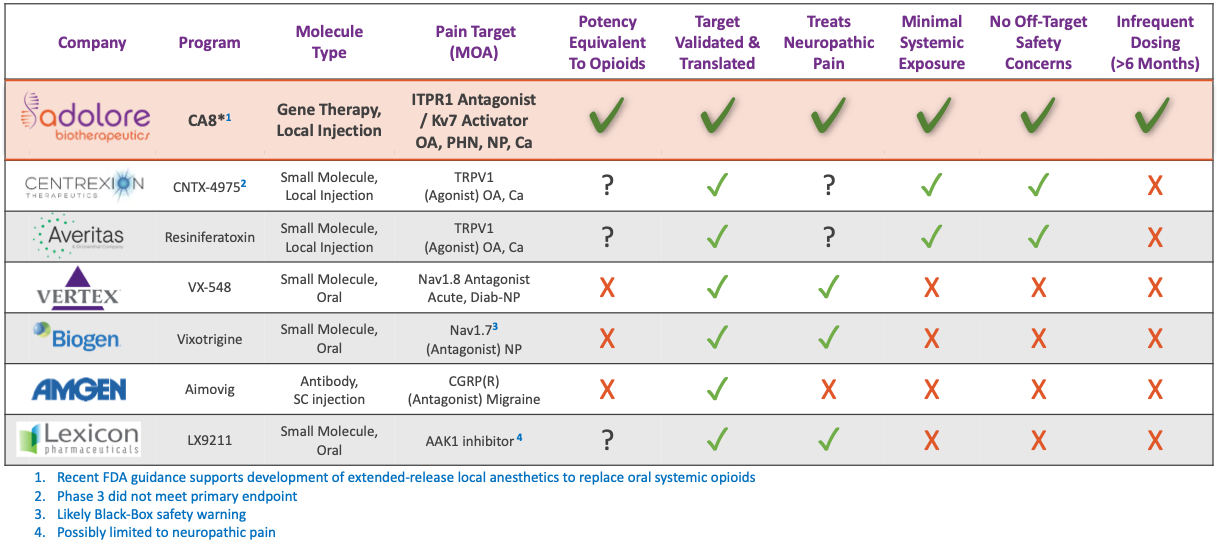
Developing Next Generation, Disease-Modifying, Non-Opioid Analgesic Gene Therapy for the Treatment of Chronic Pain
CA8*: Novel Class of Intra-Neuronal Analgesic Protein-Medications
-
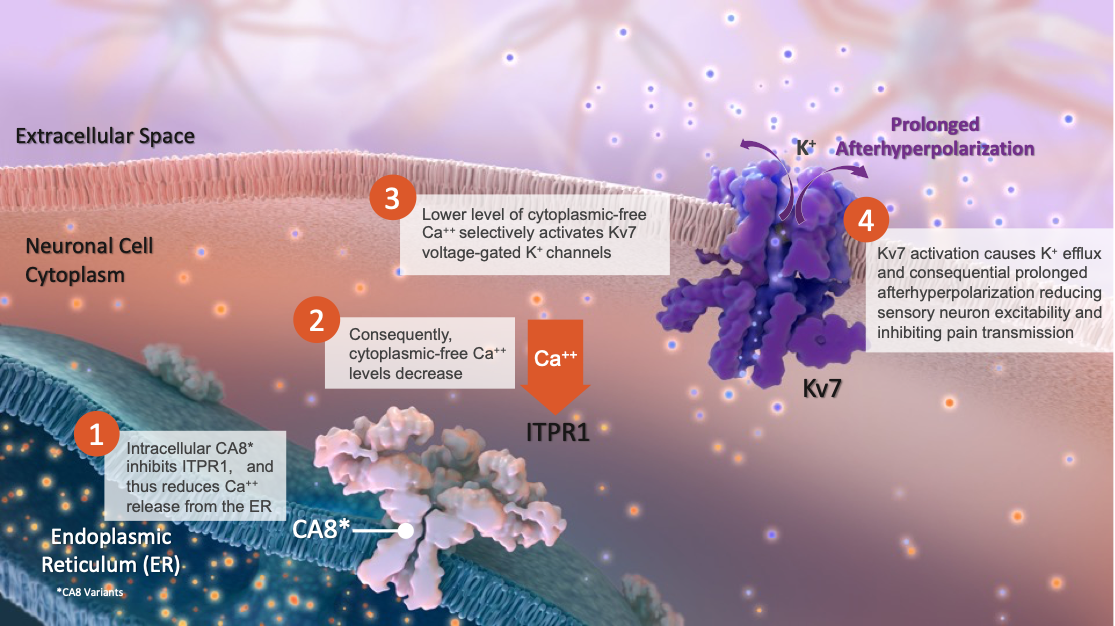
- We are advancing innovative CA8* (Carbonic Anhydrase-Like Analgesic Peptides, CA8 Variants) gene therapies for the non-opioid treatment of chronic pain. CA8* is a non-enzymatic, analgesic peptide that regulates intracellular neuronal calcium levels fundamental to pain response
- CA8* is a naturally occurring protein that is endogenously produced in the dorsal root ganglia (DRG) of sensory neurons
- Expression and function of CA8* are essential to the body’s response to external and internal pain stimuli and analgesic responses
- THE CHALLENGE: How to deliver a therapeutic protein to the inside of the targeted cells/tissue
Long-Acting CA8* Gene Therapy Delivery Inside Targeted Sensory Neuron Cells
-
- The CA8* minigene is part of our gene vector code specifying the amino acids that constitute our CA8* therapeutic protein to treat chronic pain (our vector does not integrate into the host genome and the term “minigene” is used to indicate therapeutic protein part of the gene vector code)
- Once the vector delivers the activated minigene to the targeted sensory neuronal cell, the therapeutic CA8* protein is produced inside the target cell for a prolonged period of time
- Herpes simplex virus (HSV) has demonstrated to be highly persistent in nature. We expect our precisely bio-engineered replication-defective HSV (rdHSV) vectors to enable long-term efficacy, estimated in the range of many months to several years. In our preclinical studies with a single intra-articular knee injection of rdHSV-CA8* we have observed dose-dependent efficacy that lasts for as long as 221 days (7.4 months), with a trend indicating that the ultimate efficacy is likely going to be much longer
CA8* Acts on the Kv7 Pathway, Which Does Not Involve Opioid Receptors or Pathways – Kv7 is a Proven Drug Target That Rivals the Potency of Morphine/Opioids Without the Side-Effects of Opioids and Formation of Drug-Dependence
These novel CA8* gene therapies are potent activators of Kv7, that are long-acting and locally administered with proven analgesic efficacy. They provide versatile dosing regimens and routes of administration, including intra-articular, intradermal, intra-neuronal (nerve block) and epidural injection.
High DRG CA8 Expression
Lower Pain Response
Lower DRG CA8 Expression
Greater Pain Response
Broad Applicability Across:
Neuropathic Pain
Inflammatory Pain
Nociceptive Pain
Using a replication-defective HSV (rdHSV) vector enables disease-free localized delivery to the peripheral somatosensory nervous system with an excellent safety profile. HSV vectors are known for their stability and prolonged gene expression, providing an excellent basis for long-term treatment.
Potential Across a Number of Chronic Pain Indications:
Erythromelalgia
Osteoarthritis pain
Diabetic neuropathy
Post-Herpetic neuralgia
Lower back pain
Cancer pain
Additional rare-disease
pain conditions
Validated CA8* MOA: Activation of Kv7 Potassium Channels, a Proven Pain Management Approach
Precision-Medicine: Local Administration and Tissue Specificity Limits Off-Target Effects
-
- Our rdHSV/CA8* (ADLR-1001) vector is highly efficient and specific in infecting neuronal tissue (high degree of tropism)
- We administer (ADLR-1001) at the site of pain, avoiding systemic circulation and exposure
- rdHSV does not replicate, shed, or spread, keeping exposure entirely local, only affecting sensory neurons involved in the targeted pain problem
- Intra-cellular production of CA8* inside sensory neurons avoids systemic levels that could affect other tissues
rdHSV Vector Enables Redosing of CA8* Gene Therapy
-
- rdHSV evades immune system surveillance thus enabling redosing
- Other FDA-approved HSV-based vectors have redosing regimens
- Redosing option provides a pathway toward long-term treatment for chronic pain control