Erythromelalgia as an Orphan Disease ADB-101
Erythromelalgia (EM) was originally described in 1878. The overall age- and sex-adjusted incidence rate per 100,000 people per year in a population-based study in the U.S. was 1.3 (approximately 4,000 patients) making it a rare orphan disorder. Prevalence in the U.S. is estimated at 15 per 100,000 (45,000 patients). Females are more often affected than males.

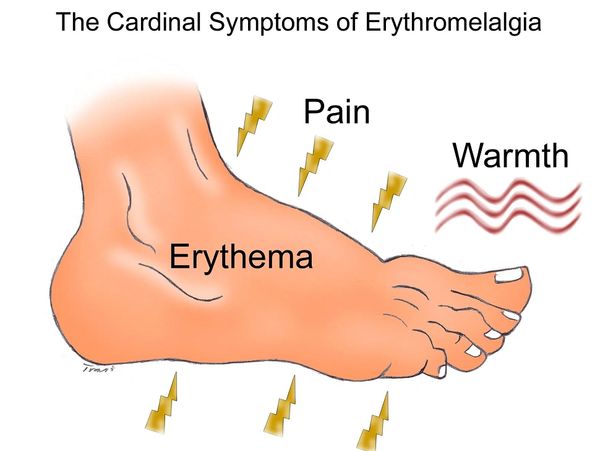
EM mainly affects the feet and less often the hands and usually both sides of the body. EM is characterized as recurrent episodes of severe pain associated with red warm feet and/or hands provoked by minimal stress (exposure to heat, exercise or anxiety). Clinical evidence suggests this disorder has a chronic course with gradual increasing severity over time. Rarely, symptoms can be almost continuous. Many affected individuals describe worsening of their symptoms during times when their lower extremities are dependent (or in the “hanging down”) position. While elevating the extremity may be helpful, these precautions may significantly negatively impact daily functioning. There are no approved therapies for EM. However, some topical medications (e.g., lidocaine that inhibits voltage-gated sodium channel functioning) may improve symptoms. In almost all cases, affected individuals may experience pain relief by cooling or immersing the affected regions in ice water.
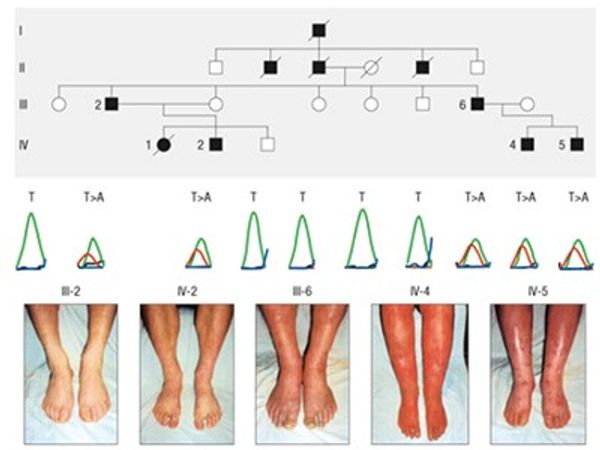
Erythromelalgia may be an isolated, primary condition or occur secondary to various underlying disorders. Numerous families (kindreds) involving affected individuals in several generations are reported. Many of these familial cases demonstrate autosomal dominant inheritance (a single copy of a mutated gene is sufficient to produce disease symptoms). Studies of families with autosomal dominant erythromelalgia most often demonstrated gain-of-function mutations in voltage-gated sodium channels critical in lowering the threshold for action potentials of pain sensory neurons (making these pain sensing neurons hyperexcitable in response to typically painless stimuli).
EM Orphan Disease Therapy
Our initial clinical development focus will be on clinical studies of a significant group of more homogenous patients with primary erythromelalgia associated with one or more genetic mutations in voltage-gated sodium channels. For our purposes, we expect to diagnose EM patients for our clinical studies by their characteristic symptoms, signs, provocation tests (hot water immersion), quantitative sensory testing, and DNA genetic mutational analyses. Related disorders that may possibly be treated with our therapeutic candidate in the future include small fiber neuropathies, complex regional pain syndrome (CRPS), musculoskeletal disorders and pain and burning from: Fabry’s, essential thrombocythemia (thrombocytosis), polycythemia vera, systemic lupus erythematosus (SLE), underlying benign tumors or malignancies, and/or other disorders and conditions with symptoms similar to EM.
ADB-101
Adolore programs are focused on localized delivery of our biotherapeutics to decrease the excitability of these nociceptive sensory neurons to control chronic EM pain. We anticipate that clinically relevant routes of administration will be available for these therapeutics including ‘nerve blocks’ and/or intra-articular joint injections. ADB-101 is a state-of-the-art JDNI8 rdHSV-CA nontoxic gene therapy designed to target sensory nerve fibers that are hyperexcitable in EM patients to relieve their chronically recurrent difficult to treat neuropathic pain.
Adolore Biotherapeutics, Inc.
13685 Whistler Mountain Road, Delray Beach, Florida 33446, United States
Copyright © 2024 Adolore - All Rights Reserved.
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.